Adverse drug reactions (ADRs) are a significant clinical problem that clinical decision support can prevent
ADRs remain a challenge in a clinical setting, particularly given the increasing complexity of therapeutics, an aging population, and rising multimorbidity. ADRs are a response to noxious and unintended drugs, which occur at doses typically used for the prevention, diagnosis, or therapy of disease or the modifications of physiological function. Clinical problemAbout 3 to 7% of all hospital admissions in the United States are due to adverse drug reactions
Adverse drug reactions occur during 10 to 20% of hospital admissions, and about 10 to 20% of these reactions are severe
Integrate medication review into your clinical workflow with Synbase
#1 clinical decision support platform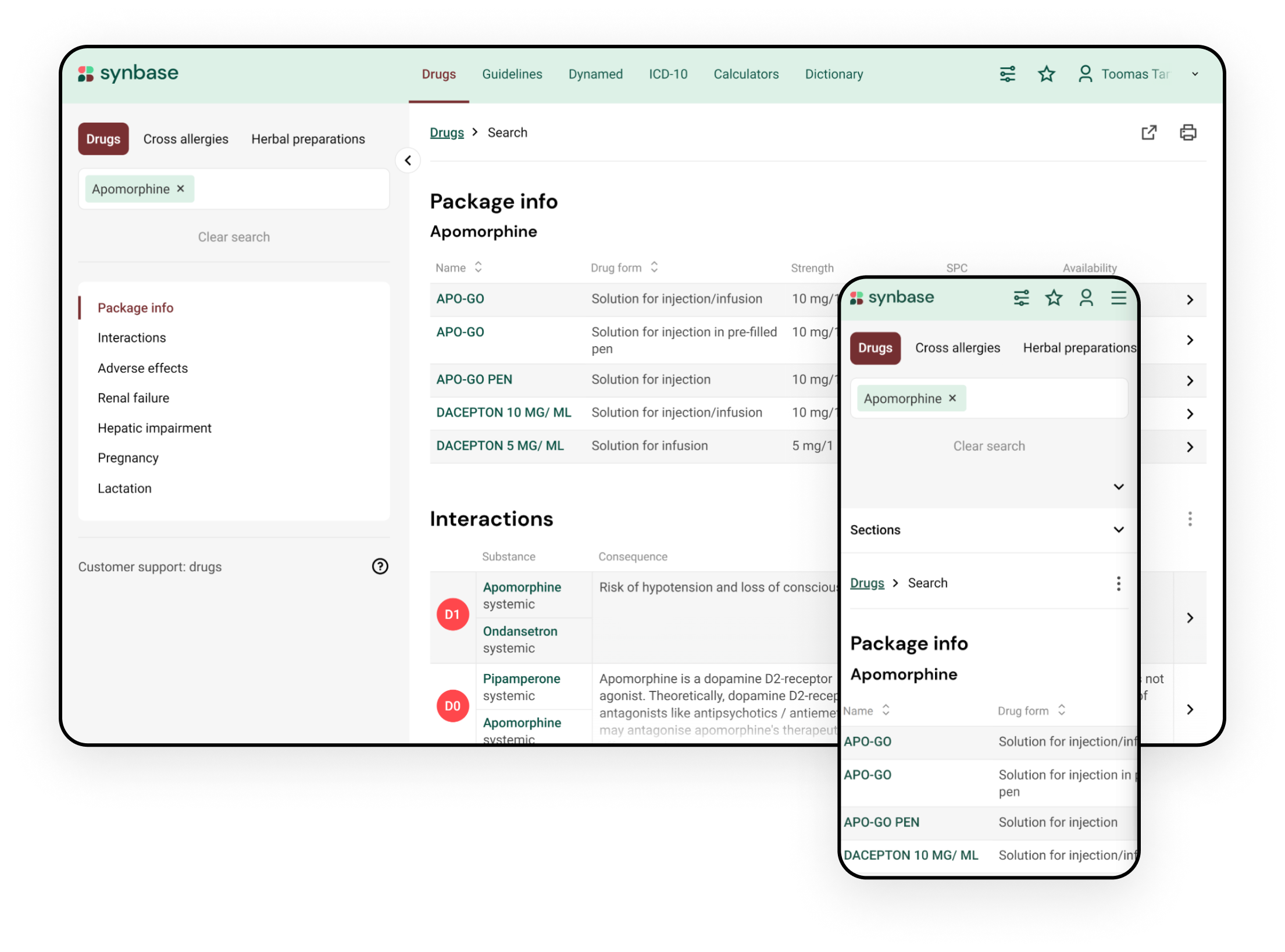
Synbase platform enables integration of medication review tools for various drug safety issues into your electronic health record system and usage of a complimentary web portal.
Medication review covers drug interactions, adverse drug reactions (ADRs), drug dosing in renal and hepatic failure, drug allergies, and drug usage during pregnancy and lactation
Evidence-based databaseMedication review based on 7 databases
Medication review promotes the safe use of drugs based on seven drug-related databases
- Drug interactions database Inxbase warns about a consequence and always provide the user with a clinical recommendation to avoid or monitor the potential drug-drug interactions. The database covers 28 000 pharmacokinetic drug interactions and considers active substance and drug administration routes.
- Riskbase is a complementary database to a drug interactions database for doctors or pharmacists presenting a risk profile of a patient’s medication based on pharmacodynamic properties. The database contains 18 000 evaluations of the risk profile of about 1500 drugs with about 11 clinically relevant adverse effects.
- Gravbase and Lactbase contain information on the safe use of drugs during pregnancy and lactation. These databases consist of data of over 1 300 drugs (prescription drugs, OTC drugs) and other substances, such as vitamin supplements, illicit drugs, caffeine, and nicotine during pregnancy and lactation.
- Renbase database includes concise, up-to-date information on the safety and detailed recommendations of 1700 drugs and other substances, such as vitamins and micronutrients in patients with renal failure. In the database, the degree of kidney failure, based on glomerular filtration rate (GFR), is divided into four categories, according to the classification by the European Medicines Agency (EMA).
- Database Heparbase provides information on the safe and effective use of 1700 drugs, including vitamins, in patients with liver diseases. In the database, the degree of hepatic impairment, based on the Child-Pugh classification, is divided into three categories, as recommended by the European Medicines Agency (EMA).
- Xreactbase covers evidence-based knowledge on cross-hypersensitivities of about 900 drugs that cause preventable drug harm.
Methodology of medication review
Drug information has been produced according to standard operating procedures (SOPs), including published medical knowledge and manufacturer-provided information approved by the European Medicines Agency (EMA) and/or The United States Food and Drug Administration (FDA) and data from national drug registers. All references are, whenever possible, linked to their source evidence.
All warnings are classified according to clinical significance (A-D), resulting in a traffic light-like system.
Regular updates for the medication review
Up-to-date information to provide medical advice – all drug databases available in the Synbase platform are developed and updated quarterly by Medbase Ltd (Finland).

