We have integrated Synbase clinical decision support platform for drug-drug interactions in several countries, including two nationwide integrations to national e-prescription systems.
Drug interactions are a significant clinical problem that can be prevented with the help of clinical decision support systems
Drug-drug interactions occur when two or more drugs react with each other. Polypharmacy is common in older patients and younger at-risk populations and increases the risk of adverse medical outcomes. Clinical problemUp to 56% of hospital inpatients are exposed to one or more potentially harmful drug combinations.
A study based on a national register in Sweden found that 3.8% of the population were given combinations of drugs classified as clinically significant D-level potential drug-drug interaction that should be avoided.
Integrate drug interaction database seamlessly into your clinical workflow with Synbase
The Synbase platform enables the integration of drug-drug interactions into your electronic health record or pharmacy information system. #1 clinical decision support platform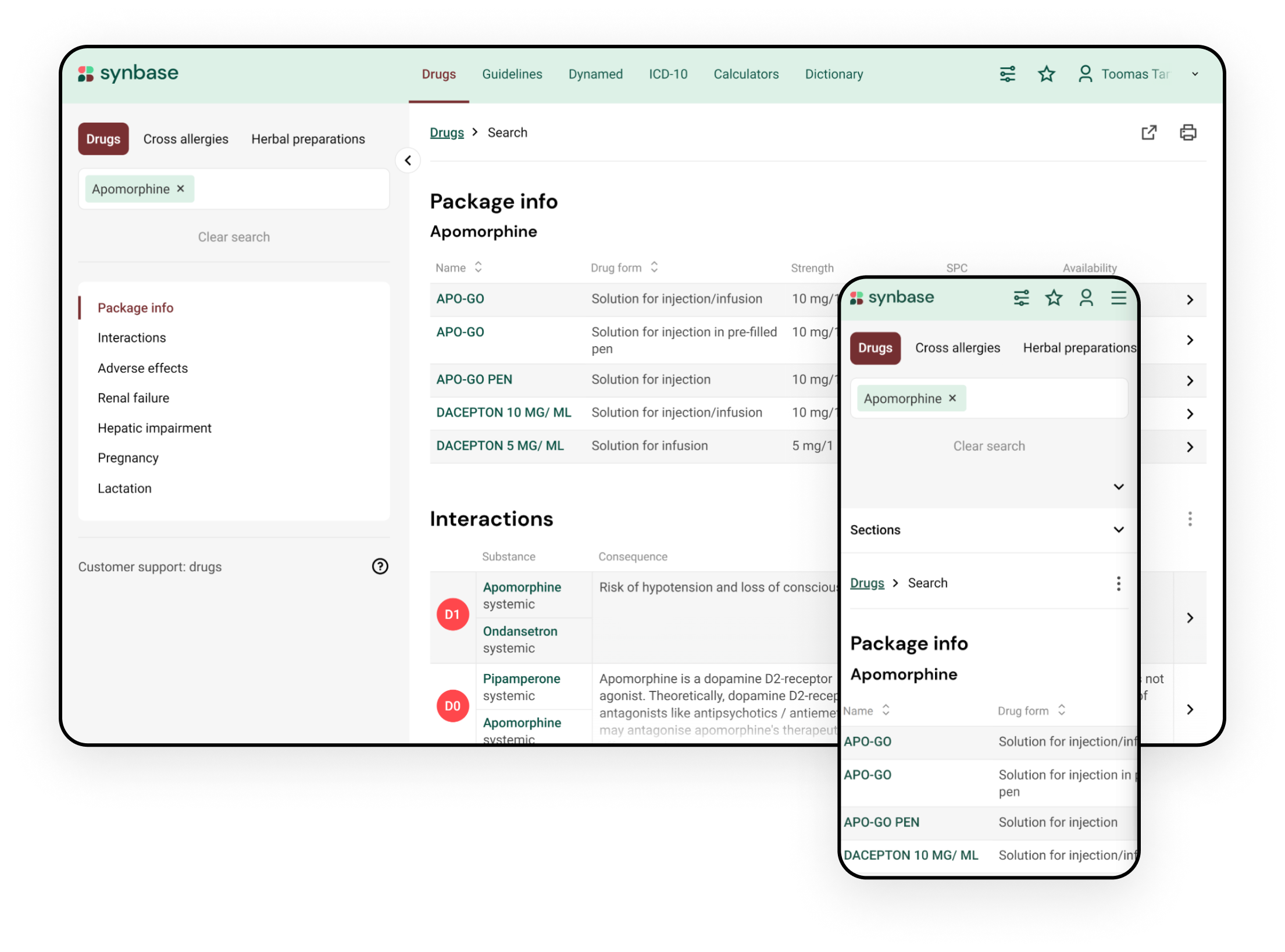
Features of Synbase platform for drug-drug interactions:
- Recommendations on avoiding or monitoring the potential drug-drug interaction
- Description of clinical consequences of drug-drug interactions in several languages
- Maps interacting drugs with local drug compendium
- Takes drug administration route into account
- Integration with other drug databases (e.g., drug dosing in renal or hepatic failure)
- Supports search with country-specific drug name
- Considers both prescription drugs and over-the-counter (OTC) drugs
- Can be used by clinicians on a desktop, tablet, or as a mobile application
Trusted by clinical organizations
Recognition-

-

Our customer Estonian Health Insurance Fund won the Estonian Association of Quality Annual Award (2016) and the European Quality Innovation Award in Social and Healthcare Innovations (2018) for drug interactions service.
Drug interactions as nationwide clinical decision support tool
Case study
Drug interactions database Inxbase includes possible drug interactions of prescription drugs, OTC drugs, and drug-food interactions
Clinical databaseDatabase on pharmacokinetic drug interactions
The drug-drug interaction database Inxbase gives warnings about potentially harmful interactions and provides the user with a clinical recommendation to avoid or monitor potential drug-drug interactions.
The database contains 28 000 pharmacokinetic drug interactions validated by clinical experts and considers active substance and drug administration routes.
The drug interaction database includes possible interactions between two or more drugs, covering prescription drugs, OTC drugs, and also drug-food interactions.
The potential drug interaction information is divided into medical consequences, recommendations, mechanisms, background, and references.
The database contains information about relevant pharmacokinetic drug interactions that are supported by scientific literature or by clinical studies referred to in the drug summary of product characteristics (SPCs) approved by the European Medicines Agency (EMA) and/or the United States Food and Drug Administration (FDA). Clinically significant pharmacokinetic interactions which can be foreseen (e.g., based on known metabolic pathways) have been extrapolated from the available evidence. All references are linked to their source evidence.
High clinical usability – all potential interactions are classified by GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology according to clinical significance (A-D), resulting in a traffic light-like system:
| A |
Minor interaction of no clinical relevance |
| B |
The clinical outcome of the interaction is uncertain and/or may vary |
| C |
Clinically relevant interaction that can be handled by, for example, dose adjustments |
| D |
Clinically relevant interaction that is best avoided |
The documentation level (0-4) of the evidence is also included:
| 0 |
Data derived from extrapolation based on studies with similar drugs |
| 1 |
Data derived from incomplete case reports and/or in vitro studies |
| 2 |
Data derived from well-documented case reports |
| 3 |
Data derived from studies among healthy volunteers and/or pilot studies among patients |
| 4 |
Data derived from controlled studies in a relevant patient population |
Complementary database on pharmacodynamic drug interactions
Riskbase is a complementary database for doctors or pharmacists that builds a personalized risk profile of a patient’s medication based on the pharmacodynamic properties.
The database contains 18 000 evaluations of the risk profile of 1 500 drugs in relation to 11 clinically relevant adverse effects:
- Anticholinergic effect
- Constipation
- Sedation
- Orthostatic effect
- Risk of bleeding
- Serotonergic effect
- Risk of seizures
- QT-prolongation
- Renal toxicity
- Potassium balance
- Sodium balance.
High clinical usability – adverse effects are classified by risk category (A-D) and risk level (0-3) supported by color-coding:
|
A |
No known pharmacological or clinical basis for an increased risk |
|
B |
There is a somewhat increased risk |
|
C |
There is a moderately increased risk |
|
D |
There is a high risk |
The interpretation of the color codes differs from Inxbase. In Riskbase, the D-level does not mean absolute contraindication but provides an indication that the overall risk is high.
Drug-drug interactions database for patients
Besides drug-drug interaction services for professional users, we can also provide a patient-related version of a drug interaction database, where warning texts covering prescription drugs and OTC medications are specially tailored for the patients. Patients’ drug interaction tools can be part of a personal health record (PHR), pharmacy information system, or mobile application.
Regular updates for drug interactions database
Up-to-date information to provide medical advice – Inxbase and Riskbase are developed and updated quarterly by Medbase Ltd (Finland).



