Drug dosage in liver failure is a considerable clinical problem in healthcare that clinical decision support can improve
In patients with impaired liver function, drug dose adjustment is often required. Non-adherence to clinical prescribing recommendations may result in severe adverse events. Clinical problemIn a retrospective study for 400 cirrhotic patients, 20% of drugs were inadequately dosed
Hospitalization of patients receiving inadequately dosed drugs, caused by preventable adverse drug reactions (ADRs), resulted in 94 additional hospital days
Integrate drug dosing tool in hepatic failure into your clinical workflow with Synbase
The Synbase platform enables the integration of drug dosing tools in hepatic failure into your electronic health record and the use of a complimentary web portal. #1 clinical decision support platform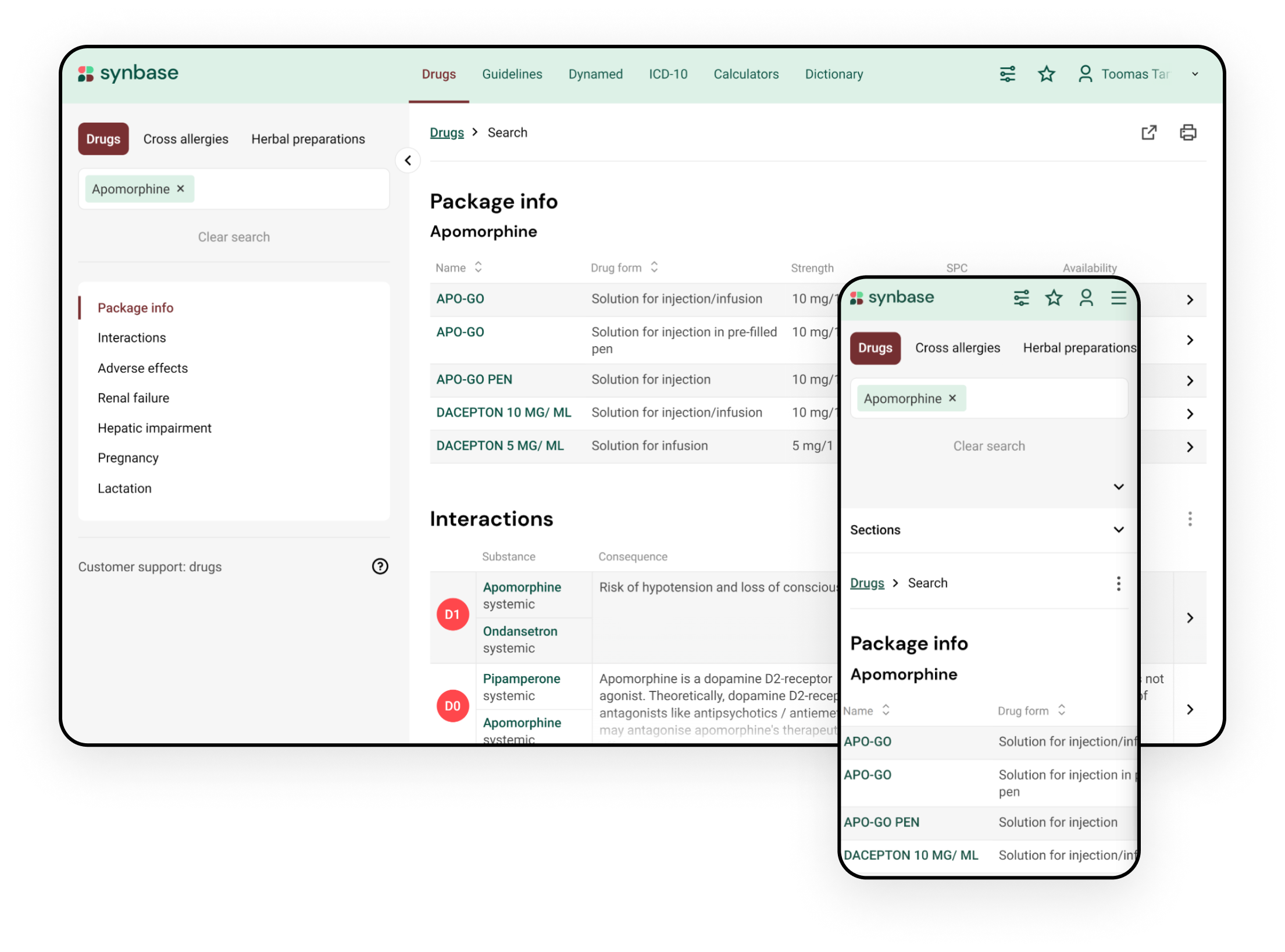
Features of Synbase platform for implementing drug dosage in hepatic failure database:
- Gives numeric recommendations for drug dosage based on the degree of hepatic failure
- Includes data on hepatoxicity of the drug
- Maps drugs with local drug compendium
- Takes drug administration route into account
- Integrated with other drug databases (e.g., drug interactions or drug warnings in pregnancy and lactation)
- Supports search with local trade name, active substance, and ATC code
- Considers both prescription and over-the-counter (OTC) medication
- Can be used in a desktop, tablet, or mobile application.
Database on drug dosing in hepatic impairment, covering prescription drugs, OTC drugs, and other substances
Clinical databaseDrug dosage in hepatic failure database
Database Heparbase provides information for patients with liver diseases on the safe and effective use of 1 700 drugs, including vitamins and supplements.
All drug information has been produced according to standard operating procedures (SOPs), including published medical knowledge and the manufacturer-provided information approved by the European Medicines Agency (EMA) and/or The United States Food and Drug Administration (FDA) and data from national drug registers. All references are linked to their source evidence.
Methodology of drug dosing in hepatic failure database
In the database, the degree of hepatic impairment, based on the Child-Pugh classification, is divided into three categories, as recommended by the European Medicines Agency (EMA):
|
Child-Pugh A (score 5-6) |
Mild hepatic impairment |
|
Child-Pugh B (score 7-9) |
Moderate hepatic impairment |
|
Child-Pugh C (score >10) |
Severe hepatic impairment |
High clinical usability – all dosing recommendations are classified according to clinical significance (A-D), resulting in a traffic light-like system:
|
A |
No need for dosage modification |
|
|
B |
The information is not available, or the recommendation is estimated based on the pharmacokinetic characteristics of the substance |
|
|
C |
Modification of the dose or dosage interval is needed |
|
|
D |
The use of this drug in patients with hepatic impairment should be avoided |
|
Regular updates for drug dosing in hepatic failure database
Up-to-date drug information to provide medical advice – Heparbase is developed and updated quarterly by Medbase Ltd (Finland).
Classification of clinical significance
High clinical usability – all dosing recommendations are classified according to clinical significance (A-D), resulting in a traffic light-like system:
|
A |
No need for dosage modification |
|
|
B |
The information is not available, or the recommendation is estimated based on the pharmacokinetic characteristics of the substance |
|
|
C |
Modification of the dose or dosage interval is needed |
|
|
D |
The use of this drug in patients with hepatic impairment should be avoided |
|

